J. Cent. South Univ. Technol. (2010) 17: 485-491
DOI: 10.1007/s11771-010-0511-7 
Thermal stability and long-acting antibacterial activity of
phosphonium montmorillonites
CAI Xiang(蔡祥), TAN Shao-zao(谭绍早), LIAO Ma-hua(廖马花),
WU Ting(吴婷), LIU Ren-fu(刘任富), YU Biao(余彪)
Department of Chemistry, Jinan University, Guangzhou 510632, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract: Na-montruorillonite (Na-MMT) was exchanged with three quaternary alkylphosphonium salts: decyl tributylphosphonium bromide (DTBPBr), dodecyl tributylphosphonium bromide (DDTBPBr) and hexadecyl tributylphosphonium bromide (HDTBPBr), to investigate the effects of phosphonium salts species and relative molecular mass on the characteristics, morphology, thermal stability and long-acting antibacterial property of phosphonium montmorillonites. The resulting modified montmorillonites were characterized by the FTIR, XRD, TEM, and TG/DTG techniques. And minimum inhibitory concentration (MIC) was used to investigate antibacterial activity. The results show that the phosphonium salts are intercalated into Na-MMT, and the basal spacing of P-MMTs is enlarged with the increase of phosphonium salt content or the growth of alkyl chain length. DDTBP-MMT-3 with 19.83% (mass fraction) of dodecyl tributylphosphonium salts, displays excellent thermal stability and long-acting antibacterial activity.
Key words: phosphonium montmorillonite; quaternary salts; surfactant; microstructure; thermal stability; long-acting antibacterial activity; water resistance
1 Introduction
Clay minerals modified with organic ions, also known as organoclays, attracted substantial attention both in fundamental research and industrial applications due to their superior multifunctional properties. Because of good swell and disperse abilities in organic solvent, organoclays were traditionally used as thickener and gellant in chemical industry fields, such as paints, lubricants, ointments, and cosmetics [1-5]. On the other hand, organoclays were also widely used in pollution control and environmental protection because of their excellent sorption capacity towards organic and non-ionic pollutants in water [6-8]. Compared with other water treatment technologies, organoclays show superior performance especially when the contaminated water contains substantial amount of oil, grease, or tannic acid [9-11].
The focus of attention over recent years, however, has been on the use of organoclays as a filler of polymers in the process of nanocomposite production because the dispersion of a small amount (<10%, mass fraction) of organoclay in the polymer matrix can dramatically improve the mechanical, thermal, gas-barrier and flame-retardant properties of polymers [12-14]. The traditional montmorillonite (MMT)-based organoclays were mainly employed by ammonium surfactants with a long alkyl chain and three short aliphatic chains or phenyl groups, which were thermo-liable (above 250 ℃) and started to degrade at nanocomposites processing temperature (200-300℃) [15]. Other cations, such as phosphonium, pyridinium and imidazolium are excellent modifiers due to their higher thermal stability. For instance, PATEL et al [16] found that phosphonium montmorillonites (P-MMTs) showed excellent thermal stability (300-400 ℃).
Moreover, based on the broad-spectrum antibacterial property of quaternary alkylphosphonium salt, P-MMTs can also be used to resist bacteria and purify water rather than to cause secondary pollution. There are few reports on studying the antibacterial activity of P-MMTs yet. Only HERRERA et al [15] reported that cetylpyridinium modified montmorillonites could facilitate the removal of bacteria from various water sources. Hence, further research is very meaningful.
The main goal of this work was to investigate the effects of phosphonium salts and relative molecular mass on the characteristics, morphology, thermal stability and long-acting antibacterial property of P-MMTs. Tributyl phosphoniums with long alkyl (decyl, dodecyl or hexadecyl) were employed as surfactants and intercalated into montmorillonite to obtain higher thermal stability P-MMTs, and the thermal stability and long-acting antibacterial activity of P-MMTs were investigated.
2 Experimental
2.1 Materials
The sodium montmorillonite (Na-MMT) rich bentonite clay with a cation-exchange capacity (CEC) of 1 mmol/g on dry basis (dried at 110 ℃) was obtained from Hongyu Clay Co., Ltd. (Zhejiang, China); decyl tributylphosphonium bromide (DTBPBr), dodecyl tributylphosphonium bromide (DDTBPBr) and hexadecyl tributylphosphonium bromide (HDTBPBr) of chemical regent grade was supplied by Qingte Chemical Industry Co., Ltd., (Shanghai, China); Mueller-Hinton broth and nutrient agar culture medium were supplied by Huankai Microorganism Co., Ltd., (Guangzhou, China); Escherichia coli (E. coli) ATCC 25922 and Staphylococci aureus (S. aureus) ATCC 6538 were supplied by Guangdong Institute of Microbiology (Guangzhou, China).
2.2 Methods
2.2.1 Preparation
P-MMTs were prepared by ion-exchange reaction. Briefly, 10.0 g Na-MMT powder and 190.0 g deionized water were mixed in a 500 mL flask and stirred at 60 ℃ for 0.5 h. To the solution, 10.0 mmol DTBPBr, 2.5, 5.0 or 10.0 mmol DDTBPBr, or 10.0 mmol HDTBPBr were slowly added under continuous stirring at 70 ℃ for 3 h to get five organoclays. Then, these modified clays were washed to be free from Br- ions tested by AgNO3 solution, dried at 65 ℃ under vacuum and then pulverized to pass through sieve with a size of 0.050 mm. The organoclays were designated as DTBP-MMT, DDTBP-MMT-1, DDTBP-MMT-2, DDTBP-MMT-3 and HDTBP-MMT, respectively.
2.2.2 Characterization
Thermogravimetric methods (TG/DTG) were conducted with a thermal analyzer (NETZSCH TG 209) under N2 flow, the temperature range of the measure- ments was 25-800 ℃ and the scanning rate was 10 ℃/min, the organic cation contents of P-MMTs were also tested by TG. X-ray diffraction (XRD) patterns of the P-MMTs were recorded on a diffractometer (D/max-1200) using the graphite monochromatic Cu Kα radiation with measurements were conducted within a 2θ range of 2.0?-30.0? at a scanning rate of 1 (?)/min; Fourier transform infrared (FTIR) spectra were measured with the Perkin Elmer Spectrum GX spectrophotometer using KBr pellet.
measurements were conducted within a 2θ range of 2.0?-30.0? at a scanning rate of 1 (?)/min; Fourier transform infrared (FTIR) spectra were measured with the Perkin Elmer Spectrum GX spectrophotometer using KBr pellet.
Transmission electron microscopy (TEM) images were obtained with CM300 TEM. The samples were obtained by cryo-ultramicrotome equipped with a diamond knife (Tecnai-20 microscope, acceleration voltage of 200 kV) at -120 ℃.
2.2.3 Antibacterial activity
The minimum inhibitory concentration (MIC) of P-MMTs against E. coli (ATCC25922) and S. aureus (ATCC6538) was measured by two-fold diluting method [17]. Briefly, P-MMTs were suspended into Mueller- Hinton broth medium to form homogeneous suspensions and then two-fold diluted into different concentrations. Each 1 mL of culture medium containing various concentrations of test sample was inoculated with 0.1 mL of 1×106 cfu/mL bacterial suspension, cultured at 37 ℃ for 24 h under shaking, and then the growth of bacteria was observed. When no growth of bacteria was observed in the lowest concentration of the test sample, the MIC of the sample was defined as this value of dilution. The test for every MIC of P-MMTs was repeated three times.
2.2.4 Desorption test
The desorption of phosphonium salts from P-MMTs was carried out by the process that 0.1 g sample was soaked in 20 mL deionized water in a polypropylene bottle at 40 ℃ on an incubator shaker. As the experi- mental time was 6, 12, 24, 48, 60 and 72 h, the released quantity of phosphonium salts from P-MMTs was measured by inductive coupled plasma (ICP) emission spectrometer, and the MIC of soaked P-MMTs against E. coli and S. aureus was also tested.
3 Results and discussion
3.1 Fourier transform infrared spectra (FTIR) analysis
In the FTIR spectra of Na-MMT (rich bentonite clay) (Fig.1), the bands at 3 500-3 750 cm-1 and near 3 430 cm-1 are indicative of montmorillonite. The broad band centered near 3 430 cm-1 is due to —OH stretching mode of interlayer water. The bands at 3 620-3 743 cm-1 are due to —OH stretching mode of Al—OH and Si—OH of montmorillonite. The bands at 3 743 cm-1 due to —OH stretching vibration may also be due to the presence of small quantity of montmorillonite. The shoulders and the broadness of —OH bands are mainly due to the contribution of several structural —OH groups occurring in this smectite. The overlaid absorption peaks at 1 630 cm-1 are attributed to —OH bending mode of adsorbed water. The characteristic peak at 1 115 cm-1 is due to Si—O stretching and out-of-plane Si—O stretching mode for montmorillonite. The band at 1 035 cm-1 is attributed to Si—O stretching (inplane) vibration for layered silicates. The FTIR peaks at 914, 877 and 844 cm-1 are attributed to Al—Al—OH, Al—Fe—OH and Al—Mg—OH bending vibration [18-19]. In the FTIR spectra of P-MMTs, the peaks at 2 925 and 2 855 cm-1 are ascribed to the asymmetric and symmetric vibration of methylene groups (CH2)n of the aliphatic chain [20-21]. P-MMTs show a weak intensity of the —OH bending vibration at 1 630 cm-1 due to adsorbed water. Therefore, the existence of the quaternary alkylphosphonium salts in the P-MMTs is proved, indicating that the phosphonium salts are intercalated into Na-MMT.
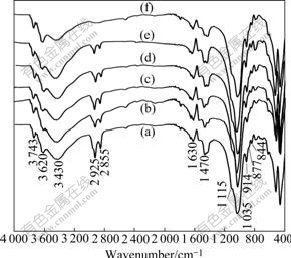
Fig.1 FTIR spectra of Na-MMT and P-MMTs: (a) HDTBP- MMT; (b) DTBP-MMT; (c) DDTBP-MMT-3; (d) DDTBP- MMT-2; (e) DDTBP-MMT-1; (f) Na-MMT
3.2 Thermogravimetric analysis
The thermogravimetric (TG) curves and derivative thermogravimetric (DTG) curves of Na-MMT and P-MMTs are shown in Fig.2. According to the mass loss in the TG curves and the prominent endothermic peaks in the DTG curves, the decomposition of Na-MMT clearly occurred in two general regions below 800 ℃ [22]: (1) the evaporation of free (absorbed) and interlayer water residing between the aluminosilicate layers and comprising the hydration spheres of the cations at 50-200 ℃; (2) the dehydroxylation of the aluminosilicate lattice between 500 and 700 ℃. In contrast, the TG and DTG curves of P-MMTs might be conveniently divided into three regions [23]: (1) the desorption of absorbed water and gases below 200 ℃; (2) the decomposition of the organic substances at 200-500 ℃; and (3) the dehydroxylation of the aluminosilicate lattice at 500- 700 ℃.

Fig.2 TG (a) and DTG (b) curves of Na-MMT and P-MMTs: 1—Na-MMT; 2—DDTBP-MMT-1; 3—DDTBP-MMT-2; 4—DDTBP-MMT-3; 5—DTBP-MMT; 6—HDTBP-MMT
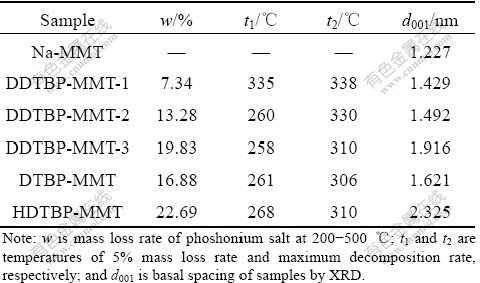
Compared with Na-MMT, the water content of P-MMTs at 50-200℃ was smaller due to the hydrophobic nature of phosphonium salts. In addition, the phosphonium salt contents of P-MMTs could be determined at 200-500 ℃, and the phosphornium salt contents (mass fraction) of DDTBP-MMT-1, DDTBP- MMT-2, DDTBP-MMT-3, DTBP-MMT and HDTBP- MMT are 7.34%, 13.28%, 19.83%, 16.88% and 22.69% (Table 1), respectively.
In this work, mass loss rate of 5% was taken as an indicator of thermal stability. Table 1 shows the strcutre and property of Na-MMT and P-MMTs. According to TG curves, significant thermal degradation temperatures of DDTBP-MMT-1, DDTBP-MMT-2, DDTBP-MMT-3, DTBP-MMT and HDTBP-MMT are 335, 260, 258, 261 and 268 ℃, respectively. Two decomposition peaks of DDTBP-MMT-1, DDTBP-MMT-2, DDTBP-MMT-3 and HDTBP-MMT in the DTG curves are between 200 and 500 ℃, and the temperatures of the maximum decomposition rate (the first peak) are 338, 330, 310 and 310 ℃, respectively. The DTG curve of DTBP-MMT exhibits three peaks and the temperature of the maximum decomposition rate (the first peak) is 306 ℃, which suggests different decomposition mechanisms. From TG and DTG curves, it can be found that P-MMTs with low concentration phosphonium salt exhibit better thermal stability for the same phosphonium salt, while P-MMTs with long-chain phosphonium salt exhibit better thermal stability for different phosphonium salts. Moreover, all the temperatures of P-MMTs at 5% mass loss rate are higher than 258 ℃, which means that P-MMTs can bear temperatures of nanocomposites processing at 200- 300 ℃.
Table 1 Structure and property parameters of Na-MMT and P-MMTs
3.3 XRD analysis
XRD patterns of Na-MMT and P-MMTs are shown in Fig.3, and the corresponding basal spacing (d001) is shown in Table 1. XRD pattern of Na-MMT displays a diffraction peak at 2θ=7.2?, which is assigned to the basal spacing of 1.227 nm. In XRD patterns of P-MMTs,
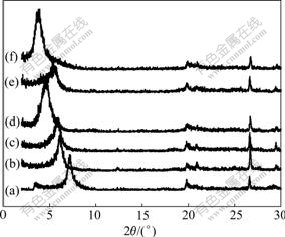
Fig.3 XRD patterns of Na-MMT and P-MMTs: (a) Na-MMT; (b) DDTBP-MMT-1; (c) DDTBP-MMT-2; (d) DDTBP-MMT- 3; (e) DTBP-MMT; (f) HDTBP-MMT
the diffraction peaks displaced to lower angle are observed, and the basal spacing varies between 1.429 and 2.325 nm. The basal spacing of P-MMTs is enlarged with the increase of phosphonium salt content or the growth of alkyl chain length.
Earlier study of montmorillonite modified with alkylammonium chlorides suggested that alkyl chains might form monolayers (1.37 nm), bilayers (1.77 nm), pseudotrimolecular layers (2.17 nm) or paraffin structure (2.20 nm) [24]. Considering that the relative molecular mass of phosphonium salt is larger than that of ammonium salt, as well as similar structure, interlayer spaces of 1.429 nm for DDTBP-MMT-1, 1.492 nm for DDTBP-MMT-2 and 1.621 nm for DTBP-MMT are consistent with a monolayer arrangement of the phosphonium salts, while that of 1.916 nm for DDTBP- MMT-3 indicates a bilayer to pseudotrilayer arrangement, and that of 2.325 nm for HDTBP-MMT suggests a paraffin structure.
3.4 TEM analysis
TEM images of Na-MMT and P-MMTs with different phosphonium salt contents and species are shown in Fig.4. All these images show that the crystal layer of the P-MMTs is agglomerated more seriously than that of Na-MMT. With the increase of the DDTBP content, the crystal layer of the P-MMTs is agglomerated greatly. While different species of phosphonium salts are employed, the agglomeration of the P-MMTs crystal layer has no difference at the same phosphonium salt content.
3.5 Evaluation of antibacterial activity
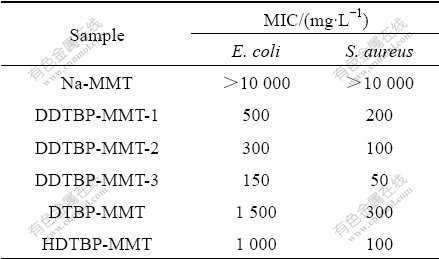
The antibacterial activity of Na-MMT and P-MMTs against E. coli and S. aureus is shown in Table 2. Na-MMT shows poor antibacterial activity because both of the MIC values are higher than 10 g/L. P-MMTs show relatively high antibacterial activity against E. coli and S. aureus, and the antibacterial activity is enhanced along with the increase of the phosphonium salt content for the same phosphonium salt. Moreover, the antibacterial activity varies with the alkyl chain length of phosphonium salts in P-MMTs. Compared with DTBP-MMT and HDTBP-MMT, DDTBP-MMT-3 reveals higher antibacterial activity of 150 mg/L and 50 mg/L MIC against E. coli and S. aureus, respectively.
In addition, P-MMTs exhibit lower activity against E. coli than against S. aureus. The structure of the cell wall of E. coli is more complicated than that of the S. aureus because another layer outside of the peptidoglycan layer i.e., outer membrane, is mainly composed of lipopolysaccharides and phospholipids.
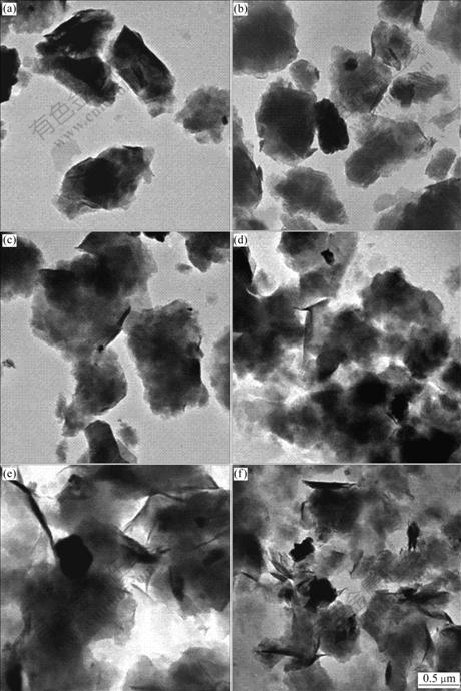
Fig.4 TEM images of different samples: (a) Na-MMT; (b) DDTBP-MMT-1; (c) DDTBP-MMT-2; (d) DDTBP-MMT-3; (e) DTBP- MMT; (f) HDTBP-MMT
Table 2 Antibacterial activity of Na-MMT and P-MMTs
Outer membrane plays a significant role in protecting bacteria cells against foreign compounds such as P-MMTs. Thus, the lower sensitivity of P-MMTs against E. coli is mainly due to the presence of the outer membrane [25].
3.6 Desorption of phosphonium salts from P-MMTs
The amount of released phosphonium salts from DTBP-MMT, DDTBP-MMT-3 and HDTBP-MMT for different soaking time is shown in Fig.5.
During the first 48 h, phosphonium salt is released quickly with the lapse of soaking time, and then released slowly. The quantities of released phosphonium salts from DTBP-MMT, DDTBP-MMT-3 and HDTBP-MMT are only 3.85%, 2.71% and 1.99% (mass fraction) until 72 h, respectively.
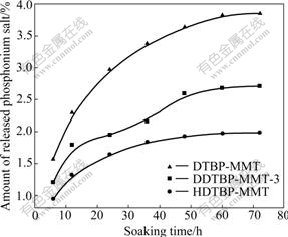
Fig.5 Amount of released phosphonium salts in saline water for different soaking time
Testing for antibacterial activity of soaked DDTBP-MMT-3 (0.9%, 40 ℃) is intended to study the effects of soaking time on the antibacterial activity of P-MMTs. The results are shown in Table 3. The MIC values of DDTBP-MMT-3 against E. coli and S. aureus show high antibacterial activity all the experimental time. And the MIC values are 300 and 200 mg/L against E. coli and S. aureus until 72 h, respectively.
Table 3 Effects of soaking time on antibacterial activity of DDTBP-MMT-3

4 Conclusions
(1) The effects of phosphonium salts and relative molecular mass on the characteristics, morphology, thermal stability and long-acting antibacterial property of montmorillonite are investigated.
(2) Thermal stability of P-MMTs is improved significantly, temperatures of all P-MMTs at 5% mass loss rate are higher than 258 ℃, and the basal spacing is enlarged with the increase of phosphonium salt content or the growth of alkyl chain length.
(3) DDTBP-MMT-3 with 19.83% of dodecyl tributylphosphonium salt, shows higher antibacterial activity with the MIC against E. coli and S. aureus of 150 mg/L and 50 mg/L. For DDTBP-MMT-3 soaked in saline water (0.9%, 40 ℃) after 72 h, only 2.71% of phosphonium salt is released, and the MIC values E. coli and S. aureus are 300 and 200 mg/L,respectively.
(4) P-MMTs display excellent thermal stability and long-acting antibacterial activity.
References
[1] GAO S L, EDITH M, ROSEMARIE P. Nanocomposite coatings for healing surface defects of glass ?bers and improving interfacial adhesion [J]. Composites Science and Technology, 2008, 68(14): 2892-2901.
[2] BURGENTZL? D, DUCHET J, G?RARD J F, JUPIN A, FILLON B. Solvent-based nanocomposite coatings: I. Dispersion of organophilic montmorillonite in organic solvents [J]. Journal of Colloid and Interface Science, 2004, 278(1): 26-39.
[3] MARKS J G, FOWLER J F, SHERERTZ E F, RIETSCHEL R L. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite [J]. Journal of the American Academy of Dermatology, 1995, 33: 212-216.
[4] CHTOUROU M, FRIKHA M H, TRABELSI M. Modified smectitic Tunisian clays used in the formulation of high performance lubricating greases [J]. Applied Clay Science, 2006, 32(3/4): 210-216.
[5] VISERAS C, AGUZZI C, CEREZO P, L?PEZ-GALINDO A. Uses of clay minerals in semisolid health care and therapeutic products [J]. Applied Clay Science, 2007, 36(1/3): 37-50.
[6] RYTWO G, TAVASI M, AFUTA S, NIR S. Adsorption of difenzoquat on montmorillonite: Model calculations and increase in hydrophobicity [J]. Applied Clay Science, 2004, 24(3/4): 149-157.
[7] CHANGCHAIVONG S, KHAODHIAR S. Adsorption of naphthalene and phenanthrene on dodecylpyridinium-modi?ed bentonite [J]. Applied Clay Science, 2009, 43(3/4): 317-321.
[8] XU Li-heng, ZHU Li-zhong. Structures of OTMA- and DODMA- bentonite and their sorption characteristics towards organic compounds [J]. Journal of Colloid and Interface Science, 2009, 331(1): 8-14.
[9] PAIVA L B, MORALES A R, D?AZ F R V. Organoclays: Properties, preparation and applications [J]. Applied Clay Science, 2008, 42(1/2): 8-24.
[10] GAO B, YANG L Y, WANG X R, ZHAO J C, SHENG G Y. In?uence of modi?ed soils on the removal of diesel fuel oil from water and the growth of oil degradation micro-organism [J]. Chemosphere, 2000, 41(3): 419-426.
[11] DENTEL S K, JAMRAH A I, SPARKS D L. Sorption and co- sorption of 1,2,4-trichlorobenzene and tannic acid by organo-clays [J]. Water Research, 1998, 32(12): 3689-3697.
[12] RAMORINO G, BIGNOTTI F, PANDINI S, RICC? T. Mechanical reinforcement in natural rubber/organoclay nanocomposites [J]. Composites Science and Technology, 2009, 69(7/8): 1206-1211.
[13] TANG Y, LEWIN M. New aspects of migration and ?ame retardancy in polymer nanocomposites [J]. Polymer Degradation and Stability, 2008, 93(11): 1986-1995.
[14] RHIM J W, HONG S I, HA C S. Tensile, water vapor barrier and antimicrobial properties of PLA/nanoclay composite ?lms [J]. LWT-Food Science and Technology, 2009, 42(2): 612-617.
[15] HERRERA P, BURGHARDT R C, PHILLIPS T D. Adsorption of Salmonella enteritidis by cetylpyridinium-exchanged montmorillonite clays [J]. Veterinary Microbiology, 2000, 74(3): 259-272.
[16] PATEL H A, SOMANI R S, BAJAJ H C, JASRA R V. Preparation and characterization of phosphonium montmorillonite with enhanced thermal stability [J]. Applied Clay Science, 2007, 35(3/4): 194-200.
[17] KASUGA N C, SEKINO K, KOUMO C, SHIMADA N, ISHIKAWA M, NOMIYA K. Synthesis, structural characterization and antimicrobial activities of 4- and 6-coordinate nickel(II) complexes with three thiosemicarbazones and semicarbazone ligands [J]. Journal of Inorganic Biochemistry, 2001, 84(1/2): 55-65.
[18] MADEJOV? J. FTIR techniques in clay mineral studies [J]. Vibrational Spectroscopy, 2003, 31(1): 1-10.
[19] TYAGI B, CHUDASAMA C D, JASRA R V. Determination of structural modification in acid activated montmorillonite clay by FTIR spectroscopy [J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2006, 64(2): 273-278.
[20] VAIA R A, TEUKOLSKT R K, GIANNELIS E P. Interlayer structure and molecular environment of alkylammonium layered silicates [J]. Chemistry of Materials, 1994, 6(7): 1017-1022.
[21] XI Y F, DING Z, HE H P, FROSR R L. Infrared spectroscopy of organoclays synthesized with the surfactant octa- decyltrimethylammonium bromide [J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2005, 61(3): 515-525.
[22] AWAD W H, GILMAN J W, NYDEN M, HARRIS R H, SUTTO T E, CALLAHAN J, TRULOVE P C, DELONG H C, FOX D M, Thermal degradation studies of alkyl-imidazolium salts and their application in nanocomposites [J]. Thermochimica Acta, 2004, 409(1): 3-11.
[23] XIE W, GAO Z M, PAN W P, HUNTER D, SINGH A, VAIA R. Thermal degradation chemistry of alkyl quaternary ammonium montmorillonite [J]. Chemistry of Materials, 2001, 13(9): 2979-2990.
[24] KOZAK M, DOMKA L. Adsorption of the quaternary ammonium salts on montmorillonite [J]. Journal of Physics and Chemistry of Solids, 2004, 65(2/3): 441-445.
[25] YOON K Y, BYEON J H, PARK C W, HWANG J. Antimicrobial effect of silver particles on bacterial contamination of activated carbon fibers [J]. Environmental Science and Technology, 2008, 42(4): 1251-1255.
Foundation item: Projects(20676049, 20871058) supported by the National Natural Science Foundation of China; Project(05200555) supported by the Natural Science Foundation of Guangdong Province, China; Projects(2007B090400105, 2008A010500005) supported by the Foundation of Enterprise-University-Research Institute Cooperation from Guangdong Province and Ministry of Education of China
Received date: 2009-09-02; Accepted date: 2009-12-16
Corresponding author: TAN Shao-zao, Professor; Tel: +86-20-85223670; E-mail:tanshaozao@163.com
(Edited by CHEN Wei-ping)