J. Cent. South Univ. Technol. (2007)04-0490-06
DOI: 10.1007/s11771-007-0095-z
Influence of polymer dispersants on dispersion stability of nano-TiO2 aqueous suspension and its application in inner wall latex paint
PENG Bing(彭 兵), HUANG Yi(黄 毅), CHAI Li-yuan(柴立元),
LI Guo-liang(李国良), CHENG Ming-ming(程明明), ZHANG Xiao-fei(张晓飞)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
___________________________________________________________________
Abstract: The effects of SN5040 and polyethylene glycol(PEG) individually and in combination on the dispersion stability of nano-TiO2 aqueous suspension were investigated by ultraviolet-visible absorption spectroscopy. The adsorption mechanism of these dispersants was detected by zeta potential, isothermal absorption and FTIR analysis. It is found that SN5040 is superior for stabilizing nano-TiO2 in aqueous suspension to PEG in basic region, and the optimum mass fraction of SN5040 addition is 3%. In the case of NaCl addition, the optimum value increases with the increase of NaCl concentration in the solution. When the mixture of SN5040 and PEG is employed, the antagonism appears preponderant. When SN5040 and PEG are added sequentially, the synergistic reaction takes place. The synergistic reaction can be attributed to the mechanism that PEG adsorption decreases the electronic repulsion between SN5040 molecules, which results in the increase of SN5040 adsorption density. PEG is adsorbed by the interaction with the pre-adsorbed SN5040 layer. Furthermore, the modified inner wall latex paint with well dispersed nano-TiO2 suspension is endowed with excellent ultraviolet absorption and antibacterial properties.
Key words: polymer dispersant; dispersion stability; nano-TiO2; inner wall latex paint; PEG adsorption
___________________________________________________________________
1 Introduction
Nano-titania is paid extensive attention due to its specific photocatalysis and widely used in plastic, coatings, ceramics and rubber. However, nano particles tend to aggregate in water and decrease its photocatalysis[1-2]. Many researches have been conducted for the influence of dispersants, especially of polyelectrolytes, on the stabilization of nano particles in water[3-5]. Polyelectrolytes adsorbed on the surface of the particles can stabilize a colloidal suspension by electrosteric function through a pure electrostatic effect of the adsorbed polymer, steric hindrance or a combination of them.
Recently, SARAVANAN et al[6-7] investigated the competitive adsorption of polyethylene glycol(PEG) and ammonium polymethacrylate (APMA) onto alumina and zirconia and its influence on dispersion stability of these micro-size particles. But the effects of mixed polymer dispersants on the stability of nano-TiO2 aqueous suspension were seldom dealt with. In this study, the dispersants of a commercial sodium salt of polyacrylic acid (SN5040) and PEG were used to stabilize nano-TiO2 in aqueous suspension individually or synchronously. The effects of the sequence of dispersants addition on dispersion stability of nano-TiO2 aqueous suspension, the mass ratio of dispersants and the salt concentration were studied. In order to investigate the mechanism of co-adsorption of these two dispersants, the results from electrokinetic analysis and dispersion stability were compared. FTIR spectroscopic analyses were carried out to determine the changes of surface chemical characteristic and judge the sort of adsorption.
Eventually, the nano-TiO2 aqueous suspensions with and without dispersants were applied to prepare inner wall latex paint respectively. The ultraviolet absorption and antibacterial properties of the paint modified with nano-TiO2 were evaluated. The results show that the key for making latex paint modified with nano-TiO2 is to disperse nano-TiO2 well in the paint.
2 Experimental
2.1 Materials
Nano-TiO2 used in this study was Degussa P25 obtained from Beijing Entrepreneur Science &Trading Co. Ltd. of China. The average diameter of the nano powders is 21 nm and the specific surface area (BET) of the powder is 46.8 m2/g.
The polymer dispersant SN5040 was supplied by Changsha Zhongcheng Paint Tech Co. Ltd. of China and the PEG was supplied by Tianjin Damao Chemical Reagent Factory in China. SN5040 is a kind of commer-cial dispersant used in latex paint and its main component is sodium salt of polyacrylic acid (Na-PAA), with acid dissociation constant (pKa) of about 4% and 50% of the carboxylic acid groups(COO-) are dissociated[8]. The average relative molecular mass of PEG (Mw) is 6 000.
Raw materials for making latex paints such as titania, kaoline, talcum powder, latex, dispersant and other auxiliary agents in this study were supplied by Changsha Zhongcheng Paint Tech Co. Ltd. of China. Sodium chloride and hydrochloric acid used in this study were of analytical reagent. Distilled water was used for all the experiments.
2.2 Methods and procedure
2.2.1 Preparation of nano-TiO2 aqueous suspension
Take 1 g of nano-TiO2 powder into a 0.25 L of Erlenmeyer flask. Mix it with 0.1 L of distilled water under an ultrasonic probe operated at full power for 30 min. Afterwards, add the dispersant solution, adjust the pH value by means of adding HCl or NaOH solution, and stir the suspension at room temperature for several hours until equilibrium.
2.2.2 PEG adsorption and desorption experiments
PEG adsorption was determined by a standard depletion method. Transfer 0.03 L of nano-TiO2 aqueous suspension mentioned above into a centrifugal tube, then centrifuge at 6 000 r/min for 30 min. Analyze the supernatant for PEG by the ammonium ferric thiocyanate aqueous-chloroform system[9].
Desorption of the adsorbed PEG from nano-TiO2 surface was assessed to evaluate the reversibility of the adsorption process. Mix 25 mL of distilled water with the residues obtained after centrifugation of the suspension containing adsorbed polymer, adjust the pH value, and then stir for 1 h. Subsequently, centrifuge and analyze the supernatant for the polymer concentration. Finally, calculate the percentage of desorption[6].
2.2.3 Dispersion stability evaluation
The dispersion stability of nano-TiO2 aqueous suspension was evaluated by the absorbance of suspension using a U-201D UV-visible spectrometer. Take 0.05 L of suspension mentioned above to a 0.05 L graduated flask. Settle it for 24 h naturally. Take 3 mL of supernatant into a 10 mm-thick spectrophotometry cell, and then detect the absorbance with the wavelength of 320 nm. The degree of absorbance is proportional to the amount of the particles per unit volume so that it denotes the dispersion stability of the particles in the aqueous solution. The absorbance of the well-stabilized suspension is ascribed to the decrease of aggregation sedimentation[10].
2.2.4 Analyses
The particle size distribution of nano-TiO2 in aqueous suspension was analyzed by a LS601 laser analyzer. Zeta potentials of nano-TiO2 particles were measured by a Delsa zeta meter. Before such measurements, mix 0.02 g of nano-TiO2 particles with 0.1 L of distilled water, settle it for 24 h naturally, adjust the pH value, add the KCl suspension of 1 mol/L to keep the ionic strength. An average of more than three measurement data for each sample was reported.
The characteristics of the interface of TiO2 and dispersants were analyzed by a nexus670 FT-IR spectrometer. Collect the TiO2 particles after being washed with distilled water and centrifuged, and then dry the powders at 120 ℃ for 6 h for FT-IR analyses.
2.2.5 Preparation of paint and its ultraviolet absorbance and antibacterial property tests
Preparation of the latex paint modified with nano-TiO2 was developed on the base of the production of normal products. Prepare the nano-TiO2 aqueous suspension with a given mass fraction, make the pigment suspension by high-speed stirring, and then slowly decant the aqueous suspension to the pigment suspension for the uniform mixture. Finally, detect the ultraviolet absorbance of paint using a U-201D UV-visible spectrometer and evaluate the antibacterial property by Shake-Flask-Test method[11] using E. coli as test strain.
3 Results and discussion
3.1 Effect of single dispersant on stability of nano-TiO2 aqueous suspension
3.1.1 Effect of pH value
Fig.1 shows the effect of pH value on the dispersion stability of nano-TiO2 aqueous suspensions with SN5040 or PEG. At pH<4.3, PEG is superior to SN5040 for stabilizing nano-TiO2 in aqueous suspension, while the result is reverse at pH>4.3. This result shows that the dispersion effectiveness of these two dispersants is affected closely by pH value. The electrical charge number on the nano-TiO2 surface and dispersants themselves will change with pH value. These changes have been reported in many earlier studies[8,10,12]. In general, the pH value of latex paint is between 7.5 and 9 so that pH value of nano-TiO2 suspension was controlled at 9 and SN5040 was used as main dispersant in following experiments according to Fig.1.
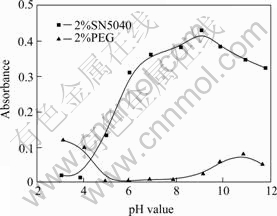
Fig.1 Effect of pH value on dispersion stability of nano-TiO2 aqueous suspension
3.1.2 Effect of SN5040 content
The effect of content of SN5040 on dispersion stabilizing of nano-TiO2 aqueous suspension is shown in Fig.2. The optimal mass fraction of SN5040 addition for stabilizing nano-TiO2 in aqueous suspension is 3% according to Fig.2. When it is over 3%, the bridging flocculation may occur among nano-TiO2 particles, which makes the stability of nano-TiO2 aqueous suspension declined. Fig.3 shows the particle size distribution of nano-TiO2 in aqueous suspension with 3% of SN5040. The particle sizes in two suspensions are in the ranges of 316-7 580 nm and 20-240 nm, and the mean diameters of them are 728 nm and 62 nm respectively. This indicates that SN5040 prevents the aggregation of nano-TiO2 in aqueous suspension effectively.
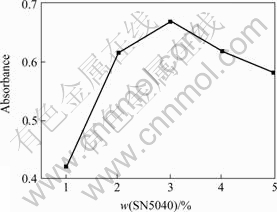
Fig.2 Effect of content of SN5040 on dispersion stability of nano-TiO2 aqueous suspension at pH value of 9
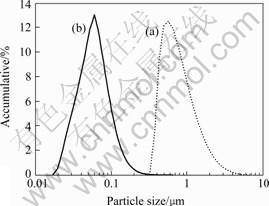
Fig. 3 Particle distribution of nano-TiO2 in aqueous suspension at pH value of 9
(a) Without dispersant; (b) With 3% SN5040
3.1.3 Effect of NaCl in aqueous suspension
The effect of NaCl on dispersion stability of nano-TiO2 aqueous suspension is shown in Fig.4. As shown in Fig. 4, the suitable content of SN5040 for stabilization improvement decreases as the increase of NaCl concentration since it screens the surface negative charge between particles and decreases the repulsive force between polyelectrolyte molecules[4]. These functions make the saturated adsorption amount of negatively charged SN5040 on nano-TiO2 surface increase and the increase of SN5040 density compensates the screened negative charge by Na+, so the suspension disperses well.
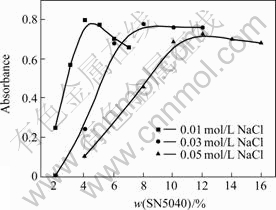
Fig.4 Effect of SN5040 content on dispersion stability at different concentrations of NaCl and pH value of 9
3.2 Combined effect of SN5040 and PEG on stability of nano-TiO2 aqueous suspension
3.2.1 Effect of sequence of adding of dispersants
In one case, SN5040 and PEG were added into nano-TiO2 aqueous suspension simultaneously and stirred for 5 h. In the other case, SN5040 was added first to the suspension and stirred for 5 h, and then PEG was added into the same suspension and stirred for 1 h. The mass ratio of PEG to SN5040 was changed and the amount of SN5040 was fixed at 2%. There presents a good correlation between dispersion stability and zeta potential in both cases (see Fig.5). This means that electronic repulsion is the dominant factor for stabilizing nano-TiO2. As shown in Fig.5(a), the absolute zeta potential increases slightly at lower PEG content, and then decreases sharply as the increase of mass ratio of PEG. Interestingly, the results shown in Fig.5(b) are opposite. These reverse results suggest that there may be a different adsorption mechanism of dispersants on solid/liquid interfaces in these two cases. When SN5040 and PEG added simultaneously, PEG may compete with SN5040 for the Ti4+ sites through the coordination between the ether oxygen of PEG or Ti4+ on nano-TiO2 surface at pH=9[6,7], which diminishes the SN5040 adsorption density so as to reduce the zeta potential. When SN5040 is added first, PEG is adsorbed on the layer pre-adsorbed SN5040 and the possible adsorption mechanism is the interaction between PEG and SN5040. This adsorption model is the same as the mixed surfactants adsorption on metal oxides reported in Refs.[13-14]. The increase of the absolute values of zeta potential in this case can be ascribed to the adsorption of non-ionic PEG, which results in the decrease of electronic repulsion among SN5040 molecules and the increase of adsorption density of negatively charged SN5040[15] . The slight decrease of zeta potential when the mass ratio exceeds 0.5 is due to the electric double layer caused by excessive PEG adsorption[10]. When PEG and SN5040 are added simultaneously, the antagonism of mixed dispersants for dispersion appears. On the contrary, the synergistic reaction works when adding SN5040 first and then PEG.
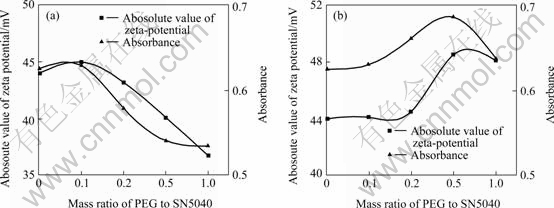
Fig.5 Effect of mixed dispersants on zeta potential and dispersion stability
(a) PEG and SN5040 added simultaneously; (b) SN5040 added first and then PEG at pH value of 9
Fig.6 shows the FTIR spectra of the original nano-TiO2 and the nano-TiO2 after SN5040 and PEG added in two different sequences at pH=9. The wave band at 3 426 cm-1 corresponding to —OH stretching vibrations shows no shift in these three spectra and it illustrates that there is no chemical interaction between PEG, SN5040 and —OH. The bands at around 1 577 and 1 411 cm-1 are attributed to stretching vibrations of —COOTi[16], which conforms that the adsorption mechanism of SN5040 is the coordination between the dissociated carboxylic acid group (—COO-) and Ti4+ Lewis acidic sites[6,7], and those at around 1 116 and 1 022 cm-1 indicate the adsorption of PEG. The bands appearing in the region from 700 to 400 cm-1 are the characteristic peaks of Ti—O stretching. It is observed that two spectra of nano-TiO2 after adsorbing dispersants are similar to the wavelength in the range from 4 000 to 700 cm-1 and different from the wavelength in the range from 700 to 400 cm-1. This provides evidence for the different mechanisms of PEG adsorption in these two cases.
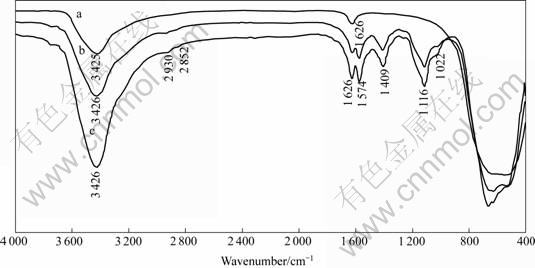
Fig.6 FTIR spectra of nano-TiO2(a), nano-TiO2 adsorbing dispersants added PEG and SN5040 simultaneously(b) nano-TiO2 adsorbing mixed dispersants added SN5040 first and PEG second(c)
3.2.2 Effect of NaCl in suspension
From the results mentioned above, it is known that the sequential addition of SN5040-first-PEG-second is beneficial for dispersion of nano-TiO2 aqueous suspension so that the mixed polymer dispersants added in this sequence under the condition of 0.03 mol/L NaCl at pH=9 are used to stabilize the nano-TiO2 aqueous suspensions.
Fig.7 shows the dispersion and electrokinetic behavior of nano-TiO2 aqueous suspension of the mixed dispersants with 0.03 mol/L NaCl and different SN5040 contents. The PEG content is independent of the dispersion stability at 8% of SN5040. The dispersion stability increases with the increase of SN5040 content at 4% of SN5040 before the mass ratio reaches 0.5 and this is the same as that shown in Fig.5(b). When SN5040 content is lower, the adsorption density of SN5040 is unsaturated so the density is enhanced in the same mechanism mentioned above, which makes dispersion stability increase. On the contrary, after SN5040 is saturated or close to saturation before PEG addition, the adsorption amount is hardly improved at higher SN5040 content due to rarity of adsorption sites (the saturation value of SN5040 is shown in Fig.4). This phenomenon is confirmed by the results of zeta potential analysis presented in Fig.7. In addition, it is observed that dispersion stability correlates well with zeta potential at both of two SN5040 contents. This means that electronic repulsion is also expected as the dominant effect for dispersion stability.
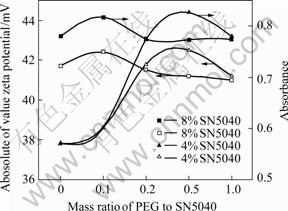
Fig.7 Dispersion and electrokinetic behavior of nano-TiO2 aqueous suspension at different mass ratio of PEG to SN5040
In order to study the adsorption behavior of PEG at two different SN5040 contents, PEG adsorption tests were carried out. Adsorption isothermals of PEG at 4% and 8% of SN5040 under 0.03 mol/L of NaCl are shown in Fig.8. These two isothermals abide by Langmuirian behavior, so it might be concluded that PEG has a monolayer adsorbed on pre-adsorbed SN5040 layer based on the characteristic of Langmuir model, which proves that PEG is adsorbed by interaction with SN5040 once again. The interaction comes from the hydrogen bonding between —OH of PEG and —COO- of SN5040. In addition, it is seen from Fig. 8 that the saturation values for these two isothermals turn out at the interval of mass ratio 0.5 and 1.0. This means that the synergistic reaction of mixed dispersants comes to peak when mass ratio of PEG to SN5040 is among this interval, which corroborates with the results of zeta potential studies.
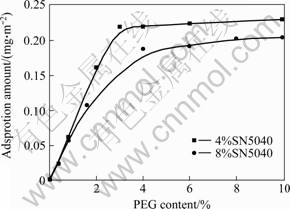
Fig.8 Adsorption isothermal of PEG in nano-TiO2 aqueous suspension with 0.03 mol/L of NaCl
3.3 Application in inner wall latex paint
Latex paints contain different ions and the pH value is high, which makes most of the ions hydrated and singly charged so 1:1 electrolyte of NaCl can be used as the representation of ionic system. The conductivity of the latex paint in this study was 0.29 S/m, so the solution of 0.03 mol/L NaCl corresponded to the ionic strength of inner-wall latex paint[17]. In order to keep the ionic strength of inner-wall latex paint constant, the nano-TiO2 suspension with 8% SN5040 and 0.03 mol/L NaCl was added to latex paint to manufacture nano-TiO2 modified paint and this paint was labeled P-C. At the same time, the paint containing no nano-TiO2(labeled as P-A) and paint added the nano-TiO2 suspension in the absence of SN5040 (labeled as P-B) were also prepared for compare. The ultraviolet absorbance and antibacterial properties were tested in 15 d and the results are given in Fig.9 and Table 1, respectively. For the paint without nano-TiO2, there was weak absorption in the range of 300-400 nm because of the background adsorption of pigmental titania and latex compared to that containing nano-TiO2. The introduction of nano-TiO2 enhances the ultraviolet absorption caused by its superior optical property. However, it can be seen that the P-C exhibits superior ultraviolet absorption property to P-B. The reason is that the increase of nano-TiO2 particle size results from aggregation and flocculation after 15 d because of no dispersants to stabilize them. The antibacterial tests have the same results as ultraviolet absorption tests. P-C has the optimal antibacterial efficiency for E. coli, which comes to 98% in ultraviolet condition due to the high effective antibacterial property of P25[18]. Hence, the dispersion stability of nano-TiO2 is the key for making nano-TiO2- modified latex paint.
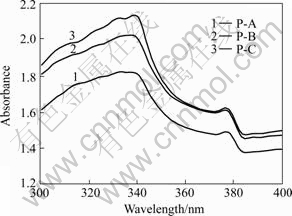
Fig.9 Ultraviolet absorption property of inner wall latex paint with and without nano-TiO2
Table 1 Antibacterial property of inner wall latex paint with and without nano-TiO2

4 conclusions
1) SN5040 is superior for stabilizing nano-TiO2 in aqueous suspension to PEG in basic region and the optimal content of SN5040 for the stabilization increases with the increase of NaCl concentration.
2) While PEG and SN5040 added simultaneously, the mixture of PEG and SN5040 is improper for dispersion of nano-TiO2. SN5040 added first and then PEG is an appropriate sequence.
3) The electronic repulsion is the dominant factor for stabilizing nano-TiO2 in aqueous suspension. PEG is adsorbed in two different mechanisms.
4) The adsorption isothermal of PEG in nano-TiO2 aqueous suspension under SN5040-first-PEG-second sequence abides by Langmuirian model, which confirms that PEG is adsorbed by the interaction with the pre-adsorbed SN5040 layer.
5) After nano-TiO2 is applied to inner wall latex paint, it gives the paint superior ultraviolet absorption and antibacterial property.
References
[1] LINSEBIGLER A L. Photocatalysis on TiO2 surface: Principles, mechanisms, and selected results[J]. Chem Rev, 1995, 95(3): 735-756.
[2] XIONG Ming-na, YOU Bo, ZHOU Shu-xue, et al. Study on acrylic resin/titaniaorganic-inorganic hybrid materials prepared by the sol-gel process[J]. Polymer, 2004, 45(26): 2967-2976.
[3] LIUFU Sheng-cong, XIAO Han-ning, LI Yu-ping, et al. Investigation of PEG adsorption on the surface of zinc oxide nanoparticles[J]. Powder Technology, 2004, 145(3): 20-24.
[4] LIUFU Sheng-cong, XIAO Han-ning, LI Yu-ping, et al. Adsorption of cationic polyelectrolyte at the solid/liquid interface and dispersion of nanosized silica in water[J]. Journal of Colloid Interface Science, 2005, 285(2): 33-40.
[5] TERAYAMA H, OKUMURA K, SAKAI K. Aqueous dispersion behavior of drug particles by addition of surfactant and polymer[J]. Journal of Colloids Surface B, 2001, 20(4): 73-77.
[6] SARAVANAN L, SUBRAMANIAN S. Surface chemical studies on the competitive adsorption of poly(ethylene glycol) and ammonium poly(methacrylate) onto alumina[J]. Journal of Colloid Interface Science, 2005, 284(2): 363-377.
[7] SARAVANAN L, SUBRAMANIAN S. Surface chemical studies on the competitive adsorption of poly(ethylene glycol) and ammonium poly(methacrylate) onto zirconia[J]. Journal of Colloids Surface A, 2005, 252 (2/3): 175-185.
[8] PETTERSSON A, MARINO G, PURSIHEIMO A, et al. Electrosteric stabilization of Al2O3 and 3Y-ZrO2 suspensions: Effect of dissociation and type of polyelectrolyte[J]. Jounal of Colloid Interface Science, 2000, 228(2): 73-81.
[9] NAG A, MITRA G, GHOSH P C. A colorimetric assay for estimation of polyethy glycol and polyethylene glycolated protein using ammonium ferric thiocyanate[J]. Analytical Biochemistry, 1996, 237(2): 224-231.
[10] LIUFU Sheng-cong, XIAO Han-ning, LI Yu-ping, et al. Polyetheylene glycol adsorption behavior on nanoparticulate TiO2 and its stability in aqueous dispersions[J]. J Inorganic Materials, 2005, 20 (2): 310-316. (in Chinese)
[11] ZHU Gui-ping, DENG Yue-quan, XU Guang-liang, et al. Applied study of antibacterial materials in coatings[J]. Chemical Building Materials, 2004(1): 9-11. (in Chinese)
[12] LOPEZ B M C, RAND B, RILEY J. Polymeric stabilization of aqueous suspension of barium titanante. Part Ⅰ: effect of pH[J]. Journal of the European Ceramic Society, 2000, 20(10): 1579-1586.
[13] WANG, W, KWAK J C T. Adsorption at the alumina-water interface from mixed surfactant solutions[J]. Colloids Surface A, 1999, 156(1/3): 175-185.
[14] WYDRO P, PALUCH M. Surface properties of cationic–nonionic mixed surfactant systems[J]. Journal of Colloids Surface A, 2004, 245(1/3): 75-79.
[15] ESUM K, NAKAIE Y, SAKAI K, et al. Adsorption of poly (ethyleneglycol) and poly (amidoamine)dendrimer from their mixtures on alumina/water and silica/water interfaces[J]. Journal of Colloids Surface A, 2001, 194(1/3): 7-12.
[16] WANG Huan-bing, LI Chun-zhong, JIANG Hai-bo. Surface modification of sub-micron antibacterial agent in organic solvent[J]. Journal of East China University of Science & Technology, 2002, 28 (6): 614-617. (in Chinese)
[17] CROLL S. DLVO theory applied to TiO2 pigments and other materials in latex paints[J]. Progress in Organic Coatings, 2002, 44(2): 131-146
[18] ALLEN S, EDGE M, ORTEGA A, et al. Degradation and stabilization of polymers and coatings: Nano versus pigmentary titania particles[J]. Polymer Degradation and Stability, 2004, 85(3): 927-946.
____________________________
Foundation item: Project(04GK2007) supported by Hunan Industrial Key Project of Science and Technology
Received date: 2006-10-20; Accepted date: 2006-12-23
Corresponding author: PENG Bing, Professor; Tel: +86-731-8830875;E-mail: pb@mail.csu.edu.cn
(Edited by ZHAO Jun)